

Vol. 38 (Nº 41) Año 2017. Pág. 22
Danieli Ledur KIST 1; Sara Isabel PÉREZ-ELVIRA 2; Luiz Olinto MONTEGGIA 3
Recibido: 06/04/2017 • Aprobado: 28/04/2017
ABSTRACT: The aim of this work was to decouple biomethanation effects of the liquid and solid fraction of Typha angustifolia biomass pretreated by thermal hydrolysis process. Pretreatment 170 ºC – 60 min present the highest solubilization of 28.6% and the increment on methane production of 51.2%. The temperature was the most influent operational condition upon the biomass solubilization. However, the increase in temperature solubilization lead to a decrease in pH to 3.95 (210 ºC at 30 min), resulting in an adverse operating condition effect upon the microorganisms community and a decrease on the biomethanization parameters. |
RESUMO: O objetivo deste trabalho foi dissociar os efeitos biometançãos da fração líquida e sólida da biomassa Typha angustifolia, pretratada pelo processo de hidrólise térmica. Pré-tratamento 170 º c – 60 min apresentam o maior solubilização de 28,6% e o incremento na produção de metano de 51,2%. A temperatura era a condição operacional mais influente sobre a biomassa solubilização. No entanto, o aumento da temperatura solubilização levar a uma diminuição do pH a 3,95 (210 º c a 30 min), resultando em um efeito de condição de funcionamento adverso sobre a comunidade de microrganismos e uma diminuição dos parâmetros biometanização. |
Thermal hydrolysis pretreatment is a technique widely used to accelerate the organic matter biodegradability on anaerobic digestion (ABELLEIRA-PEREIRA et al., 2015; FDZ-POLANCO et al., 2008; PEREZ-ELVIRA; FDZ-POLANCO; FDZ-POLANCO, 2010). The anaerobic process has two limitation steps: the first one, the enzymatic hydrolysis, and the last one, methanogenesis (SHAHRIARI et al., 2012).
The enzymatic hydrolysis starts the process and has the function to convert complex organic matter into simple organic matter, like polymers to monomers. This molecular substrate conversion is carried out by a group of microorganisms that brake down (hydrolyze) the substrate by exoenzymes, thereby allowing their cellular absorption (ANGELIDAKI et al., 2011; COSTA et al., 2013). Therefore, the hydrolysis step of the anaerobic digestion could be considered the main limiting step of the process, especially for substrates with a high content of particulate biomass (CHANDRA et al., 2007). As an example, carbohydrates hydrolysis takes place in a few hours, proteins and lipids in a few days, whereas lignocellulose and lignin are enzymatic hydrolyzed slowly and incompletely (DEUBLEIN; STEINHAUSER, 2010).
Studies suggest two important effects of the pretreatment that improve the efficiency of the hydrolysis step on anaerobic digestion. One is the solubilization of the organic matter by pretreatment process. Thermal pretreatment conditions (time, temperature/pressure and steam explosion) breakdown polymers, releasing monomers and other degradation products in a liquid medium formed by the solubilized matter and the steam condensation. The second effect is the increase in the surface area of the solids due to the biomass solubilization and the breakdown of fiber structures, like lignin (GUPTA; TUOHY, 2013; TAHERZADEH; KARIMI, 2008).
The soluble organic matter could increase the biodegradable fraction of the substrate, as the substrate is easily available for microorganism’s absorption. On this case, the substrate can skip the enzymatic hydrolysis step of the anaerobic digestion, going directly to the acidogenic (second) or even acetogenic (third) step, depending of the substance formed during the process (WANG et al., 1999). Whereas, the enhancement of the surface area, and particle size reduction exposes the solid biomass structure, providing microorganisms attack on the particulate substrate and increasing the biomethanation due the increment on enzymatic hydrolysis step of the AD process (GUPTA; TUOHY, 2013; TAHERZADEH; KARIMI, 2008). However, this biomethanation increment upon thermal hydrolysis pretreatment are contestable, as it depends on the biomass type and characteristics, and especially as a consequence of the biodegradability of the soluble material released (PANAGIOTOPOULOS et al., 2011). In short, for lignocellulose biomass it is fundamental to analyze the biodegradability route of substrate phases (liquid and solid) for biomethanation, in order to evaluate effects and efficiency of thermal hydrolysis pretreatment (BOLADO-RODRÍGUEZ et al., 2016).
Typha angustifolia, also named as narrow-leaved cattail, is an emergent macrophyte used for phytoremediation in constructed wetlands (VYMAZAL, 2013). Due it emergent characteristic, this plant had a resistant biomass structure and the intrinsic characteristic of present leaves like a spongy structure near the stem, difficult it biodegradability (PAEPATUNG; NOPHARATANA; SONGKASIRI, 2009). On this way, considered this peculiar characteristics and aiming search an alternative energy for this lignocellulosic biomass, the goal of this work is decouple pretreatment effects on liquid and solid phase, evaluating pretreatment conditions, solubilization, biomethanation and kinetic parameters of Typha angustifolia biomass pretreated upon a wide range of thermal hydrolysis conditions.
Typha angustifolia was collected at Esgueva River, in the city of Valladolid (Spain). The plants were in healthy appearance and aerial part, approximately 10 cm above the water surface, was selected for this experiment. In this case, the submerged part of the plant was discarded due to the very large and thick roots, impeding the laboratory processing of samples.
The biomass was stored under refrigeration less than three days before being processed. One day before the pretreatment assays, the plants were crushed in a domestic mill until an average particle size of 1 to 2 cm. In addition, of the particle size reduction, the crushed process ensured the biomass homogeneity.
The fresh biomass was characterized by elemental analysis, performed to determine the content of carbon, hydrogen, nitrogen and sulfur. This analysis was performed in an elemental microanalyzer LECO CHNS model 932. Also the solid fraction (total solids and volatile solids), chemical oxygen demand (total and soluble) and pH of all samples (raw and pretreated biomass) were determined following the Standard Methods (APHA, 2005).
Thermal hydrolysis (TH) pretreatment was performed to 150 g of biomass, in a 5 L reactor heated with steam from a boiler. Different temperature/pressure (vapor-liquid equilibrium) and time combinations were assessed (140 ºC, 170 ºC and 210 ºC; 5 min and 30 min). Pretreated biomass presented two phases, liquid and solid, which were separated through sieving (#1 mm) for 10 min. Therefore, all the pretreatment conditions generated a soluble and a particulate biomass fraction. Figure 1 shows the solid fraction of the biomass after pretreatments.

Figure 1. Solid phase of narrow-leaved cattail after pretreatment conditions
at 140 ºC – 5 min (A), 170 ºC – 5 min (B) and 210 ºC – 5 min (C).
BMP tests were performed in triplicate in 120 mL serum bottles, filled with 40 mL of the mixture of substrate and anaerobic inoculum (fresh digested sludge of the municipal WWTP of Valladolid) at a substrate to inoculum ratio (SIR) of 0.5 gVS gVS-1 (NEVES; OLIVEIRA; ALVES, 2004) and supplemented with micro and macronutrients, sodium bicarbonate and sodium sulfite (FERREIRA, 2013). The bottles were incubated in a thermostatic chamber at 35 ºC, and continuously mixed by a horizontal shaker. Biogas production was periodically monitored by pressure measurements, and the biogas composition was followed by chromatography (FERREIRA, 2013).
The anaerobic digestion evaluation was performed by equations (Table 1), applied to obtain key parameters of the process. It is worth to mention that the solubilization factor was calculated in relation to the tCOD and sCOD, being the sCOD determined by a filtration methodology, according with the Standard Methods.
Table 1. Equations applied to evaluate the pretreatment and biomethanation
parameters of raw and pretreated substrate
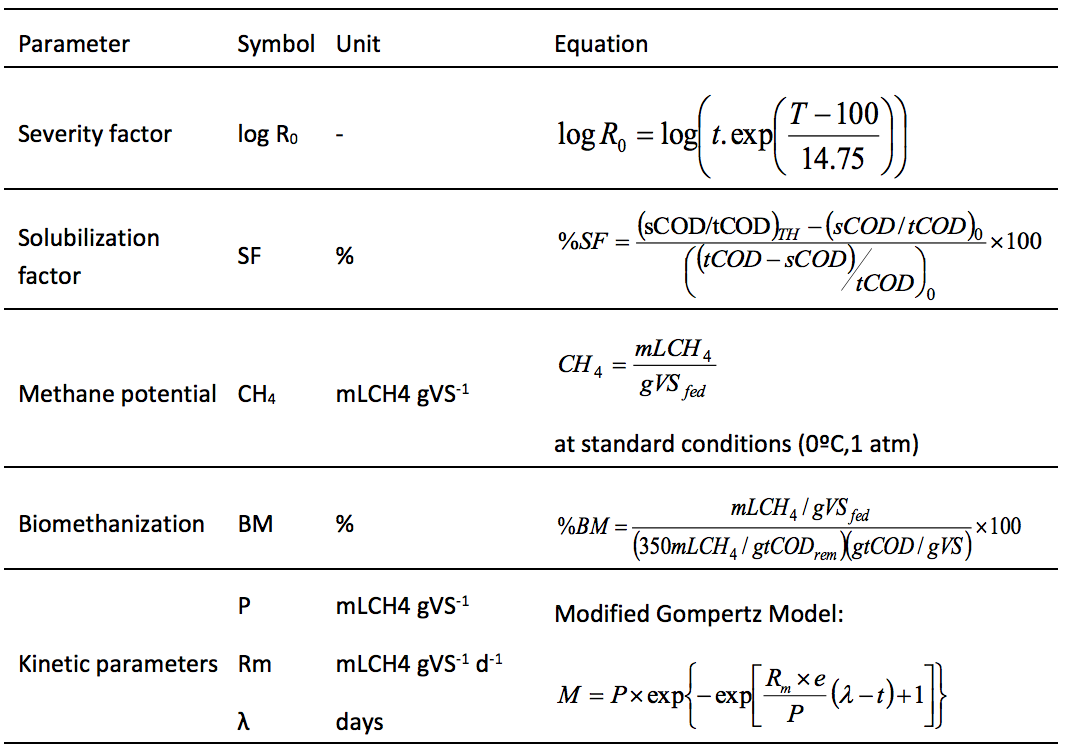
t,time; T, temperature; P, maximum methane production;
Rm, maximum methane production rate, λ, lag-phase.
The results were presented on average values and evaluated by analysis of variance (ANOVA), on a confidence level of 95%, and Pearson’s correlation coefficients, using Microsoft Excel®.
The biomass characterization was performed to compare the pretreatments effects on the biomass composition and to contribute for the evaluation of the biomethanation process. The C/N ratio found for the raw biomass was 29/1, considered in the range (from 20 to 30) of the optimal relation for anaerobic digestion (CHANDRA et al., 2007). Hence, this C/N ratio, a priori, is not prone to present inhibitory effects on the microorganism’s consortia of anaerobic digestion system.
Table 2 shows severity factor (log R0) of pretreatments performed, values of solids (total and volatile) and total COD of the raw and pretreated biomass, also the soluble COD and pH of liquid phase samples. These parameters are presented in terms of content, and not consider the mass balance, to best characterize and evaluate the substrate. The raw biomass was considered as a complete particulate substrate, hence it was not taken into account the possible release of soluble organic matter under normal conditions. In this way, all the material present in the liquid fraction was evaluated as result of the pretreatment solubilization.
Table 2. Substrate characterization of Typha angustifolia of raw, and solid and liquid fraction
of thermal hydrolysis pretreatment conditions in terms of solid, organic matter and pH content

logR0, severity factor; TS, total solids; VS, volatile solids; tCOD, total chemical
oxygen demand; sCOD, soluble chemical oxygen demand.
Mass balance, consider the dilution factor (data not shown), of total solids exhibited an increase of the soluble biomass with the increment in values of temperature and time. According to the table, the severity factor of 1.9 presented the lower increment of solids released to the liquid fraction (9.5%). The higher increment was 31.4% for the severity factor of 3.8. An expressive increment on TS released was observed when the pretreatment temperature increased from 140 ºC to 170 ºC. In addition, a lower increment was observed when the temperature increased from 170 ºC to 210 ºC, as consequence of a notable reduction on TS solubilization, especially in the pretreatment 210 ºC at 60 min, with increased only 1.9% on relation to the sample 210 ºC at 30 min. The temperatures 140 ºC and 210 ºC presented a significant difference on the biomass released (P = 0.009) for this operational condition. O relation of cooking time condition, pretreatments at 170 ºC revealed the more expressive increment of solids in the liquid phase, from 12.0% at 5 min reaction to 31.4% at 60 min reaction. The operational condition 210 ºC also increased the soluble fraction of total solids in the bulk, however lower than the pretreatment 170 ºC. The severity factor of 3.9 presented 20.8% of TS increase in the bulk and 28.4% for severity factor of 5.0.
Cooking time presented low effect than temperature for TS release in the thermal hydrolysis pretreatment of Typha angustifolia. Nitsos, Matis & Triantafyllidis (2013) related a pronounced xylan removal from biomass with increasing temperature (from 130 to 220 ºC) for severity factor higher than 3.5 on thermal hydrolysis pretreatment compared to time increment, in this study the author evaluate a time range from 15 to 180 min. These values agree with ours results and could explain the solubilization increment through the temperature aggressiveness.
In the same way, the effect of temperature values (140, 170 and 210 ºC) upon the apparent size (Figure 1) of the solid phase obtained at 5 min cooking time evidence the positive effect of the temperature to avoid the degradability, due to a substantial modification of the substrate structure. In the first condition (140 ºC for 5 min), it is still possible to identify almost perfectly the biomass structure, including the remaining green color feature characteristic of the narrow-leaved cattail leaf. In this second aggressiveness condition, it is evident the particle size reduction, as resulted of fibers breakdown. Finally, the intensification on the fibers breakout, increasing expressively the particle size reduction in this last and more aggressive temperature of pretreatment.
According to Mshandete et al. (2008), the pH variation of the substrate is related to the volatile fatty acids (VFAs) production due the degradation of the lignocellulose biomass on fermentation metabolism. In this study, the pH decreased according to the increment on pretreatment aggressiveness, starting at 5.44 for the 1.9 severity factor to 3.95 for 4.7 severity factor. Evidencing the change on pretreated substrate pH by the severity factor increment.
Polysaccharides (cellulose and hemicelulose) are degraded to other monosaccharides such as glucose, xylose, arabinose and by-products such as levulinic, acetic acid and formic acid on pretreatment of lignocellulose biomass. By-products are produced on a secondary degradation, resulting in the pH decrease of pretreated medium (NITSOS; MATIS; TRIANTAFYLLIDIS, 2013). Contrary to these evidences, the pretreatment condition at 210 ºC - 60 min (greater severity factor) increased the pH value in relation to the pretreatment at 210 ºC - 30 min, the increment was from 3.95 to 4.32. The retraction in the increase of solids in the liquid fraction and the respective pH increment between pretreatments of 210 ºC - 30 min and 210 ºC - 60 min could be explained by the volatilization of soluble organic compounds. According Nitsos et al. (2013), the time increment is more effective than temperature on intermediary degradation products from monosaccharides (like furfural) on the solubilized material. Theoretically, about 30% of the total C5 sugars could be converted in furfural and thus to volatile organic acids, being feasible their loss by volatilization (GIBSON et al., 2012; NITSOS; MATIS; TRIANTAFYLLIDIS, 2013). Moreover, considering the TS reduction, dissolved lignin can recondensate on the surface of the particulate biomass for pretreatment conditions greater than 150 ºC and on acid pH (ALVIRA et al., 2010). Therefore, the reduction in the soluble organic matter on the most severe pretreatment (210 ºC – 60 min) could be a consequence of the volatilization of by-products and the recondensation of the dissolved lignin on the solid fraction as a direct consequence of the process aggressiveness.
Theoretical methane yield (NmLCH4 gVS-1) was determined from the performed substrate characterization (FERREIRA, 2013) on raw, liquid and solid samples of Typha angustifolia, were as follows: 471.9 mLCH4 gVS-1 for raw, 491.3 mLCH4 gVS-1 for liquid phase, and 484.4 mLCH4 gVS-1 for solid phase. Increments on tCOD/VS ratio increased the theoretical methane yield of the pretreated samples, shown an increment on coefficient of specific organic matter conversion to COD of pretreated samples (AQUINO; SILVA; CHERNICHARO, 2006).
Pretreatment effects on composition and characteristics of liquid phase substrate are important factors to be evaluated for methane production by anaerobic digestion process. Table 3 show parameters of pretreatment performance and kinetic of raw and liquid phase pretreated biomass of Typha amgustifolia.
Table 3. Performance parameters obtained for raw and liquid phase
of Typha angustifolia thermal hydrolysis pretreated

logR0, severity factor; SF, solubilization factor; BM, biomethanization factor;
P, maximum methane production; Rm, maximum methane production rate;
λ, lag-phase; R2, coefficient of determination.
Solubilization factor increased clearly with the pretreatment aggressiveness, also as discussed previously by solid released with the pretreatments. However, for pretreatments as 210 ºC the SF decreased. The high solubilization factor was 28.6% for sample 170 ºC – 60 min. Also, biomethanization factor follow the same behavior, being observed frequent instabilities on samples pretreated with severity factors higher than 2.8. The higher BM factor was 88.2% for sample 210 ºC – 5 min, representing an increment of 26.1%, as compared by the raw biomass.
The maximum methane production rate (Rm) of the raw substrate was 16.7 mLCH4 gVS-1 d-1, being this parameter increased for all pretreated samples. The lower temperature of pretreatment (140 ºC) presented the higher increments on methane production rate, being the methane production rate average of 53.3 mLCH4 gVS-1 d-1 for this condition. This parameter reduced with the pretreatment temperature increment, for the pretreatment condition of 210 ºC the maximum methane production rate average was 33.5 mLCH4 gVS-1 d-1. Sewage sludge also improved the maximum methane production rate especially at low severity conditions of thermal pretreatment (ORTEGA-MARTINEZ et al., 2016). In addition, trends of lag phase reduced significantly for the pretreatments at low severity conditions and increased with the aggressiveness. On this work, raw biomass present a lag-phase of 14.8 days, being completely eliminated at 140 ºC of operational conditions and presented an average of 1.5 days at 210 ºC of operational conditions. The reduction on the efficiency of maximum methane production rate and lag-phase with the temperature/aggressiveness increment are reported as the formation of more complex and recalcitrant compounds (FERREIRA et al., 2014; ORTEGA-MARTINEZ et al., 2016).
Regarding the time influence, the kinetics of methane production from the anaerobic digestion process did not presented significant variation. Ferreira et al. (2013) found similar trends pretreating wheat straw without phase separation. This author reported the best operational condition for biomass biodegradability as 220 ºC – 1 min. These results indicate the importance of the temperature on the reaction and the negligible influence of the cooking time.
Figure 2 exhibits the initial pH values and the increase of maximum methane production (P) and the maximum methane production rate (Rm) in relation to the raw biomass, regarding the pretreatment conditions.

Figure 2. pH values, and increase on maximum methane production (P) and maximum
methane production rate (Rm) of the liquid phase samples on relation to the raw substrate
of Typha angustifolia biomass.
A slope down was observed for Rm and pH values with the increment of the process aggressiveness, mainly due to the operating temperature. The positive correlation (0.87) between the Rm and the pH of the samples evidenced an adverse operational condition, probable inhibition effect, caused by pH on the system. The lignocellulose biomass degradation at high aggressiveness conditions produced acids as by-products of monosaccharides degradation, leading to a pH decrease (ZHANG et al., 2008)(ZHANG et al., 2008). The activity of methanogenic microorganisms is considerably impaired at pH values less than 6.5 (LEITAO et al., 2006)(LEITAO et al., 2006). The pH toxicity may be associated with the presence of undissociated volatile fatty acids (VAN LIER et al., 2001; ZHANG et al., 2008)(ZHANG, ZENG, et al., 2008; VAN LIER, TILCHE, et al., 2001), fact that explains the Rm decrease for samples with low initial pH values. The increase of Rm in the sample 210 ºC – 60 min may be related to the volatilization and dissociation of organic compounds, and the consequent increment of the pH on the bulk. Moreover, the reduction of the organic matter content due to volatilization explain the decrease on biomethanization factor. The operational condition 210 ºC – 60 min was the low biomethanization factor of the pretreated samples, with 80.1% compared to 88.2% of the substrate 210 ºC – 5 min, the best one.
Figure 3 presents the cumulative methane profile (BMP tests) obtained for raw and pretreated samples. Raw sample produced 292.8 mLCH4 gVS-1, representing a conversion of 62% of the biomass to methane, consider the theoretical methane yield of the sample. As can be observed, all the pretreated substrates had a positive effect considering the methane production increase and the lag-phase reduction comparing to the untreated (raw) substrate. The higher increment in methane production was 51.3% for the pretreatment condition 170 ºC – 60 min, representing a conversion of 90.2% of the biomass to methane, consider the theoretical methane yield of the sample.

Figure 3. Cumulative methane production of raw and liquid
phase of thermal hydrolysis pretreated biomass of Typha angustifolia.
Final methane production resulted in similar values for all pretreated samples, although the expressive fragmentation of the biomass due to the pretreatments, as observed in the Figure 1, which agree with the increment of the solubilization results presented on Table 3. These results demonstrates that the biomethanation process of the soluble fraction are not correlated to the increment of the pretreatment aggressiveness, due to the complex increment on the chemistry degradability of the organic matter and formation of more complex and recalcitrant compounds, especially at high temperature conditions (FERREIRA et al., 2014; ORTEGA-MARTINEZ et al., 2016). The biomass degradability is a result of two mechanisms: the increment on the easily biodegradable substrate and the decrease due to the thermal biomass degradation to soluble inhibitors and volatile compounds (STUCKEY; MCCARTY, 1984).
The slope increment on methane content in the biogas (Figure 4) follow the same tendency of the maximum methane production rate. The pretreatment condition at 140 and 170 ºC reached the maximum methane content (70%) in approximately 5 days of incubation, whereas samples 210 ºC at 5 and 30 min required approximately 8 days of incubation to reach this amount. Sample 210 ºC at 60 min follow the trend of samples pretreated at 140 and 170 ºC, reaching also 70% on methane content on biogas in 5 days. This result corroborate with the previous discussion, evidencing the volatilization of VFA on the sample 210 ºC at 60 min.

Figure 4. Percentage of methane content in the biogas produced on
BMP assays by the raw and liquid phase of thermal hydrolysis
pretreated biomass of Typha angustifolia.
All the pretreated samples presented a fast exponential and stability on the profile of methane content, as consequence of the easily biodegradable substrate content. However, the raw sample presented two inflection points of production. The inflection point is probably due to the depletion of a specific substrate (YASUI; GOEL, 2010)(YASUI; GOEL, 2010). So, the results evidence that the first stage of methane production, from the initial incubation period to 5 days are related to the consumption of the easily biodegradable biomass and the second, from 15.6 to 24 days, related to the slow degradable biomass of the raw substrate. The constant high methane content evidenced the high biodegradability of the liquid phase substrate pretreated by thermal hydrolysis process.
Effects of thermal hydrolysis pretreatment on methane production of solid phase substrate of lignocellulose biomass could be better understood by performance and kinetics parameters of anaerobic digestion process. Table 4 shown parameters of pretreatment performance and kinetics of raw and solid phase pretreated biomass of Typha angustifolia.
Table 4. Performance parameters obtained for raw and solid phase
of Typha angustifolia thermal hydrolysis pretreated
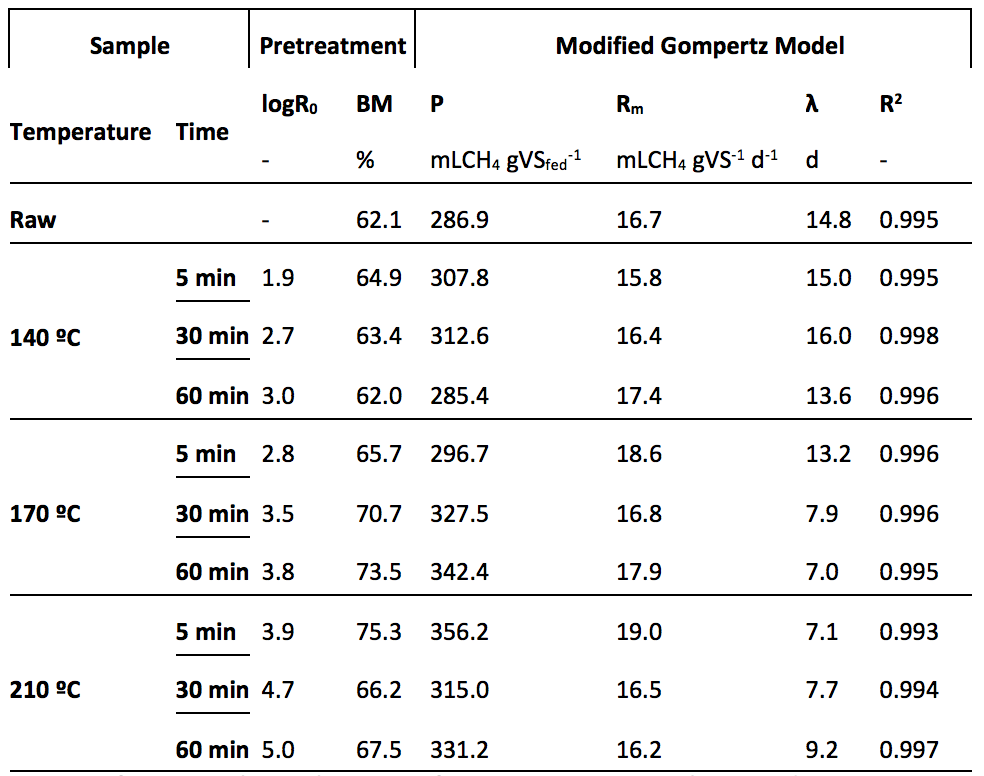
logR0, severity factor; BM, biomethanization factor; P, maximum methane production;
Rm, maximum methane production rate; λ, lag-phase; R2, coefficient of determination.
Also for the liquid phase, the biomethanization factor was higher for sample 210 ºC – 5 min, reach 75.3%, an increment of 13.2% on relation to the raw substrate. Biomethanization either shown instabilities behavior on pretreated samples. Samples pretreated at 140 ºC reduced the biomethanization with time increment. However, thus pretreated at 170 ºC increased them with time increment. In addition, at 210 ºC, the high BM factor was at 5min (75.3%), reducing to 66.2% at 30 min and again increase to 67.5% at 60 min.
The maximum methane production rate (Rm) presented a not significant increment on relation to the raw substrate, as observed for liquid phase samples. The higher rate was 19.0 mLCH4 gVS-1 d-1 for sample 210 ºC – 5 min. As expected, the solid substrate presented a wide lag-phase for all substrates, as compeer to the liquid phase, due to their particulate form that required a long retention time for enzymatic hydrolysis (ANGELIDAKI et al., 2011). The high lag-phase reduction, compared to the raw biomass, was to 7.0 and 7.1 days for substrates 170 ºC – 60 min and 210 ºC – 5 min, respectively. However, the samples 140 ºC – 30 min and 140 ºC – 5 min presented an increment on lag-phase, in relation to the raw substrate, of 1.2 and 0.2 days, respectively. The increment on temperature of pretreatment shown positive effect on lag-phase parameter (P=0.017) of the solid fraction, in opposition with the lag-phase results obtained for liquid fraction samples.
The increase in the maximum methane production, maximum methane production rate and lag-phase of solid fraction substrate on relation to the raw biomass (Figure 5), show a tendency of increase the methane production, and the lag-phase reduction in relation to the raw substrate upon the pretreatments aggressiveness between 2.8 to 3.9, for pretreatments at 170 ºC and 210 ºC. This aggressiveness range indicate the best conditions to pretreat Typha angustifolia for anaerobic digestion, resulting in the bests kinetic parameters.
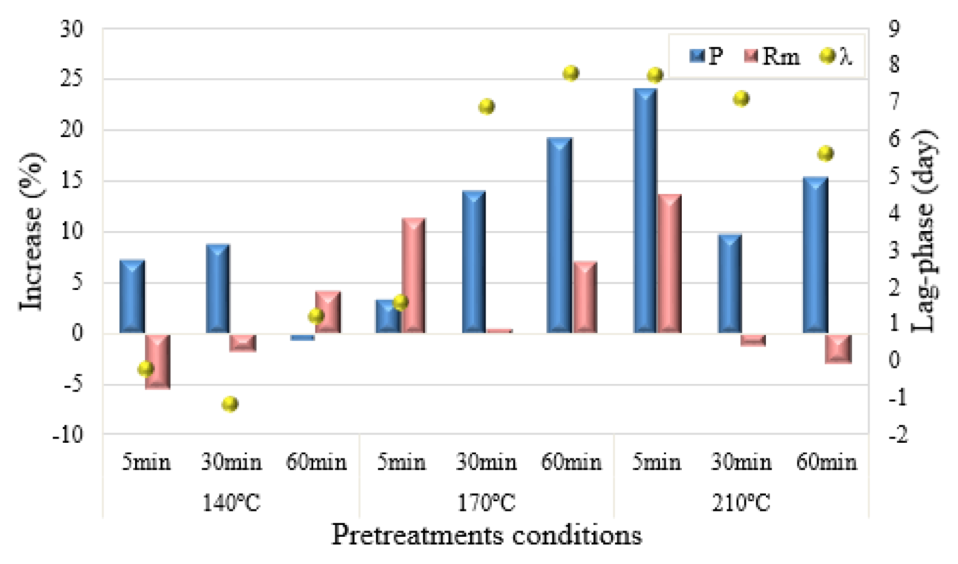
Figure 5. Increase on maximum methane production (P), maximum methane
production rate (Rm) and lag-phase of the solid phase samples on relation
to the raw substrate of Typha angustifolia biomass.
The results obtained indicated that the pretreatments conditions of 170 ºC – 60 min and 210 ºC – 5 min provided the most favorable conditions to enhance the biomethanization of the solid fraction of Typha angustifolia. It is evident the influence of the pretreatment aggressiveness on the porosity and surface area increase of the biomass, as observed by Ruangmee & Sangwichien (2012)Ruangmee & Sangwichien (2012) testing the same biomass plant with NaOH pretreatment process for enzymatic hydrolysis. Also a biomethanization decrease was observed on the solid fraction of wheat straw and sugarcane bagasse pretreated at aggressiveness condition of thermal and acid pretreatments due the release of inhibitory compounds (BOLADO-RODRÍGUEZ et al., 2016)(BOLADO-RODRÍGUEZ et al., 2016).
The regression results of all kinetic parameters for the substrates pretreated at operational conditions 210 ºC – 30 min and 210 ºC – 60 min probably indicate the high content of recalcitrant materials in these particulate substrates. The pretreatment aggressiveness allowed the solubilization of the accessible carbohydrates, remaining a recalcitrant substrate (DEUBLEIN; STEINHAUSER, 2010)(DEUBLEIN; STEINHAUSER, 2008), resulting in metabolic limitation of methane production due the low biomass biodegradability (ZHANG et al., 2014). In a way that for light pretreatments, special thus pretreated at 140 ºC, the pretreatment effect was short, increasing the porosity and surface area with the increment of the aggressiveness, and, consequently, kinetic parameters increments. Zhang et al. (2014) also obtained kinetic reduction for yard waste pretreated at 121 ºC – 30 min, comparing to the untreated substrate. This author found a positive methane production only after 45 days of digestion. Evidencing the negative effect of low temperatures of thermal hydrolysis pretreatment on lignocellulose biomass.
The biomethanation profile of the solid fraction obtained by the pretreated narrow-leaved cattail is shown in Figure 6. The negligible kinetic effects of pretreatments at 140 ºC could be observed on the graphic, all this sample profile follow the raw sample values. Sample 140 ºC – 60 min produced 296 mLCH4 gVS-1, being an increment of 1.1%. Sample 210 ºC – 5 min had the higher methane production, 376 mLCH4 gVS-1, for solid phase samples. Representing a conversion of 77.6% of the biomass to methane, consider the theoretical methane yield of the sample, and an increment of 28% of volume of methane produced by the raw substrate.
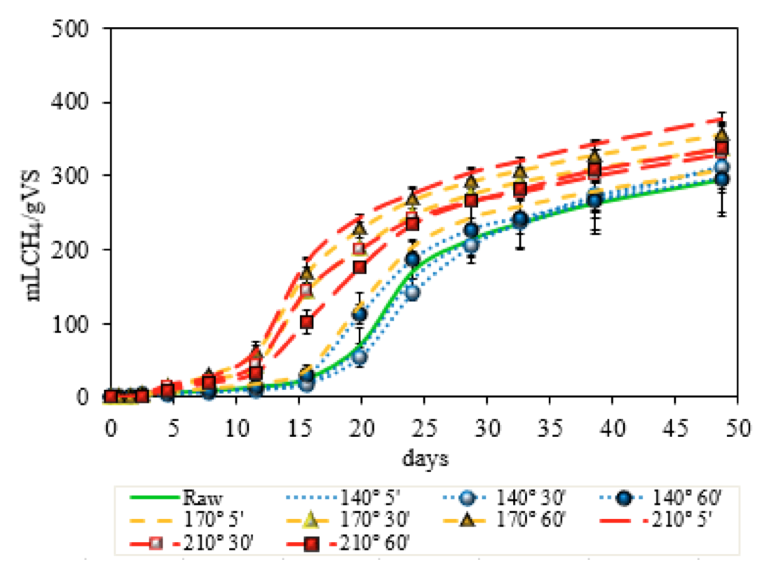
Figure 6. Cumulative methane production of raw and solid phase
of thermal hydrolysis pretreated biomass of Typha angustifolia.
Regarding the methane content in biogas production, all the samples presented inflection points (Figure 7). The first inflection point was approximately at day 2 of incubation, due to the easily biodegradable biomass retained on the biomass structure. The second inflection point evidenced a change on the specific substrate, changing from biodegradable substrate to a slow biodegradable (YASUI; GOEL, 2010), and a new adaptation of the inoculum to the substrate available. The slope increment on the methane production at 210 ºC reveal the improvements and benefits due to the increase on the surface area of the substrate obtained by the pretreatment.
After the first stage, it is noticeable the distinct effect of the pretreatment temperatures on the methane content in biogas. The samples pretreated at 210 ºC provided a fast increase, with the best exponential increment in methane content, reaching the maximum amount (approximately 70%) after 15 days of incubation. Whereas the samples of 140 ºC reached the maximum methane content after 20 days.

Figure 7. Percentage of methane content in the biogas produced on BMP assays
by the raw and solid phase of thermal hydrolysis pretreated biomass of Typha angustifolia.
Few works decoupling solid and liquid fraction of substrates of thermal hydrolysis pretreatment effects for anaerobic digestion process are available in the specialized literature. Many studies are related to fermentation system for alcohol or acids production as a biorefinery process, without inferences of the pretreatment effect upon methane production. Therefore, this study evaluated the biomethanation process of lignocellulose biomass after thermal hydrolysis pretreatment decoupling the soluble and particulate substrate behavior on mesophilic condition.
Typha angustifolia presented an efficient response on thermal hydrolysis pretreatment for methane production. The temperature was the most influent parameter on the biomass solubilization. However, the increase on the aggressiveness resulted in higher rate of organic matter degradation, leading to the reduction of the pH due to the acid formation on the bulk as by-product of the process. The higher biomethanization factor was found for pretreatment 210 ºC – 5 min for both substrate phases, increasing 26.1% for liquid and 13.2% for solid phase. High kinetic increments were obtained for all liquid phases of the pretreated samples, special maximum methane production and lag-phase. For solid phase samples, the kinetics parameters were lower and eventually negative. The bests increments on methane production was for pretreatment 170 ºC - 60 min for liquid phase (51%), and 210 ºC – 5 min for solid phase (28%). However, the variation of the final methane production was not expressive when comparing the values obtained among the pretreated samples due the long time of incubation.
As expected, the increment on methane production of the liquid phase was consequence of the organic matter solubilization. However, for pretreatments at 210 ºC occurred volatilization of VFA and recondensation of dissolved lignin, reducing the organic matter content on the bulk and increasing pH values.
The improvement in methane production of the solid phase was related to the surface area increment of the particulates, increasing the substrate availability for the enzymatic hydrolysis. Two inflection points on the profile of methane content were observed for the solid samples, resulted of depletion and change on the substrate source, more specifically, from easily biodegradable substrates to slow biodegradable substrates.
Considering the expressive variation of the biodegradability time of the soluble and particulate substrate, evaluate the separation of the soluble and particulate substrate for the design of real scale biomethanation processes are recommended, also to ensure appropriate values of the retention time for full-scale applications and a robust and stable operation of the reactors.
The authors express their gratitude to CNPq Agency for the doctorate scholarship and CAPES Funding Agency, Ministry of Education of Brazil, for the sandwich doctorate scholarship at the University of Valladolid by process number 12770/13-2.
ABELLEIRA-PEREIRA, J. M. et al. Enhancement of methane production in mesophilic anaerobic digestion of secondary sewage sludge by advanced thermal hydrolysis pretreatment. Water Research, v. 1, p. 330–340, 2015.
ALVIRA, P. et al. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresource technology, v. 101, n. 13, p. 4851–61, jul. 2010.
ANGELIDAKI, I. et al. Biomethanation and Its Potential. In: CR, A.; WR, S. (Eds.). . METHODS IN ENZYMOLOGY. 1. ed. Waltham: PubMed, 2011. p. 329–353.
APHA. Standard Methods for the Examination of Water and Wastewater. 21. ed. Washington: American Public Health Association, 2005.
AQUINO, S. F. DE; SILVA, S. DE Q.; CHERNICHARO, C. A. DE L. Considerações práticas sobre o teste de demanda química de oxigênio (DQO) aplicado a análise de efluentes anaeróbios. Engenharia Sanitária e Ambiental, v. 11, n. 4, p. 295–304, 2006.
BOLADO-RODRÍGUEZ, S. et al. Effect of thermal, acid, alkaline and alkaline-peroxide pretreatments on the biochemical methane potential and kinetics of the anaerobic digestion of wheat straw and sugarcane bagasse. Bioresource Technology, v. 201, p. 182–190, 2016.
CHANDRA, R. P. et al. Substrate pretreatment: the key to effective enzymatic hydrolysis of lignocellulosics? In: OLSSON, L. (Ed.). . Biofuels. 1. ed. New York: Springer-Verlag Berlin Heidelberg, 2007. v. 108p. 67–93.
COSTA, C. et al. Biomethanation Potential of Biological and Other Wastes. In: GUPTA, V. K.; TUOHY, M. G. (Eds.). . Biofuel Technologies. 1. ed. Berlin: Springer-Verlag Berlin Heidelberg, 2013. p. 369–396.
DEUBLEIN, D.; STEINHAUSER, A. Biogas from Waste and Renewable Resources. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA, 2010.
FDZ-POLANCO, F. et al. Continuous thermal hydrolysis and energy integration in sludge anaerobic digestion plants. Water science and technology : a journal of the International Association on Water Pollution Research, v. 57, n. 8, p. 1221–6, jan. 2008.
FERREIRA, L. C. et al. Influence of thermal pretreatment on the biochemical methane potential of wheat straw. Bioresource Technology, v. 143, p. 251–257, 2013.
FERREIRA, L. C. et al. Thermal steam explosion pretreatment to enhance anaerobic biodegradability of the solid fraction of pig manure. Bioresource Technology, v. 152, p. 393–398, 2014.
FERREIRA, L. C. G. Evaluación De La Biodegradabilidad Anaerobia De Residuos Orgánicos Pre-Tratados Térmicamente. [s.l.] Universidad de Valladolid, 2013.
GIBSON, L. T. et al. Measurement of volatile organic compounds emitted in libraries and archives: an inferential indicator of paper decay? Chemistry Central journal, v. 6, n. 42, p. 1–22, 2012.
GUPTA, V. K.; TUOHY, M. G. Biofuel Technologies. 1. ed. Berlin, Heidelberg: Springer Berlin Heidelberg, 2013.
LEITAO, R. et al. The effects of operational and environmental variations on anaerobic wastewater treatment systems: A review. Bioresource Technology, v. 97, n. 9, p. 1105–1118, 2006.
MSHANDETE, A. M. et al. Effect of aerobic pre-treatment on production of hydrolases and volatile fatty acids during anaerobic digestion of solid sisal leaf decortications residues. African Journal of Biochemistry Research (AJBR), v. 2, n. 5, p. 111–119, 2008.
NEVES, L.; OLIVEIRA, R.; ALVES, M. M. Influence of inoculum activity on the bio-methanization of a kitchen waste under different waste/inoculum ratios. Process Biochemistry, v. 39, n. 12, p. 2019–2024, 2004.
NITSOS, C. K.; MATIS, K. A.; TRIANTAFYLLIDIS, K. S. Optimization of Hydrothermal Pretreatment of Lignocellulosic Biomass in the Bioethanol Production Process. ChemSusChem, v. 6, n. 1, p. 110–122, jan. 2013.
ORTEGA-MARTINEZ, E. et al. From pre-treatment toward inter-treatment. Getting some clues from sewage sludge biomethanation. Bioresource Technology, v. 212, p. 227–235, 2016.
PAEPATUNG, N.; NOPHARATANA, A.; SONGKASIRI, W. Bio-Methane Potential of Biological Solid Materials and Agricultural Wastes. As. J. Energy Env., v. 10, n. 1, p. 19–27, 2009.
PANAGIOTOPOULOS, I. A. et al. Effect of pretreatment severity on the conversion of barley straw to fermentable substrates and the release of inhibitory compounds. Bioresource Technology, v. 102, n. 24, p. 11204–11211, 2011.
PEREZ-ELVIRA, S. I.; FDZ-POLANCO, M.; FDZ-POLANCO, F. Increasing the performance of anaerobic digestion: Pilot scale experimental study for thermal hydrolysis of mixed sludge. Frontiers of Environmental Science & Engineering in China, v. 4, n. 2, p. 135–141, 22 abr. 2010.
RUANGMEE, A.; SANGWICHIEN, C. Evaluation of Enzymatic Saccharification of Lignocellulose from Narrow Leaves CattailThe 10th International PSU Engineering Conference. Anais...2012
SHAHRIARI, H. et al. Anaerobic digestion of organic fraction of municipal solid waste combining two pretreatment modalities, high temperature microwave and hydrogen peroxide. Waste management (New York, N.Y.), v. 32, n. 1, p. 41–52, jan. 2012.
STUCKEY, D. C.; MCCARTY, P. L. The effect of thermal pretreatment on the anaerobic biodegradability and toxicity of waste activated sludge. Water Research, v. 18, n. 11, p. 1343–1353, 1984.
TAHERZADEH, M. J.; KARIMI, K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: a review. International Journal of Molecular Sciences, v. 9, n. 9, p. 1621–1651, set. 2008.
VAN LIER, J. B. et al. New perspectives in anaerobic digestion. Water Science and Technology, v. 43, n. 1, p. 1–18, 2001.
VYMAZAL, J. Emergent plants used in free water surface constructed wetlands: A review. Ecological Engineering, v. 61, p. 582–592, 2013.
WANG, Q. et al. Degradation of volatile fatty acids in highly efficient anaerobic digestion. Biomass and Bioenergy, v. 16, n. 6, p. 407–416, 1999.
YASUI, H.; GOEL, R. Application of Mathematical Models to Anaerobic Digestion Process. In: FANG, H. H. P. (Ed.). . Environmental Anaerobic Technology: Applications and New Developments. 1. ed. Hong Kong: Imperial College Press, 2010. p. 420.
ZHANG, P. et al. Anaerobic co-digestion of biosolids and organic fraction of municipal solid waste by sequencing batch process. Fuel Processing Technology, v. 89, n. 4, p. 485–489, 2008.
ZHANG, Z. et al. Impact of pretreatment on solid state anaerobic digestion of yard waste for biogas production. World journal of microbiology & biotechnology, v. 30, n. 2, p. 547–54, fev. 2014.
1. Institute of Hydraulic Research, Federal University of Rio Grande do Sul, Bioprocess and Biotechnology Engineer. Mail: danielikist@yahoo.com.br
2. Department of Chemical Engineering and Environmental Technology, University of Valladolid Chemical Engineer. Mail: sarape@iq.uva.es
3. Institute of Hydraulic Research, Federal University of Rio Grande do Sul, Mechanical Engineer. Mail: montegia@iph.ufrgs.br