Espacios. Espacios. Vol. 30 (2) 2009. Pág. 26
Desiree Moraes Zouain * y Marco Antonio Grecco D’Elia**.
Recibido: - Aprobado
It is an exploratory and qualitative survey about technical barriers faced by Brazilian small and medium companies that are electrical medical equipment producers when exporting to European Union. It looks for the main difficulties faced by these companies, to identify if there are real technical barriers imposed by European Union or if there are “pseudo technical barriers” (technical difficulties faced by the producer to adequate its product or production process to the buyer market demands, and to show this adequation, either if the demands are officials, established by a government, or if established by the consumers).
The survey questionnaire was developed & administered in such way to group in five dimensions the main variables, considered in this study, for the success of the electrical medical equipments exportation process to European Union, correlating with the difficulties faced, as follows:
The selection of the companies to be surveyed considers the following criteria: the size (small and medium companies), the innovation effort and exportation capacity, and the technological content of the products. Ten producers (Brazilian companies) were invited and seven among them participated effectively.
3.2.1 Dimension I – Company and product characteristics
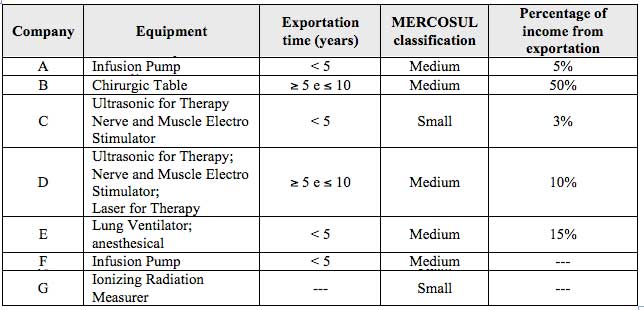
This dimension characterizes the main products, the capacity against MERCOSUL criteria, the percentage of income from exportation, and the time since began to export (Table 2).
Table 2
Company and product characteristics

Source: Prepared by the authors
It looks for the innovations that facilitate the exportation. Most of the answers mentioned the products adequation to obtain the CE mark. The specific innovations mentioned were:
3.2.2 Dimension II – Brazilian and European markets requirements
In this dimension, the survey obtained the main technical standards and regulations in Brazil and European Union that the products have to be in accordance (Table 3).
Table 3.
Standards and regulations to electrical medical equipments in Brazil and European Union

Source: Prepared by the authors
The tests applied to certificate the Brazilian equipments are shown on Table 4. It can be mentioned that the accredited laboratories by INMETRO are accepted by the European Union authorities.
Table 4
Equipment tests and laboratories
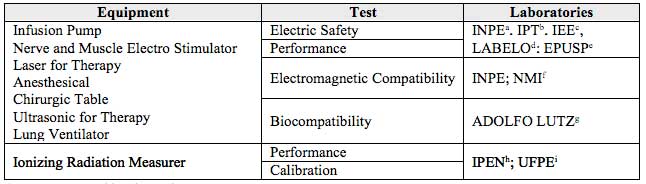
Source: Prepared by the authors
a National Institute for Space Research
b Institute for Technological Research
c Energy and Electrotechnical Institute of University of São Paulo
d Electric/Electronic, Calibration and Tests Specialized Laboratories of Catholic University
e Polytechnic School of São Paulo University – Biomedical Engineering Laboratory
f NMI Brasil Ltda.
g Adolfo Lutz Institute
h Nuclear and Energetic Research Institute i Federal University of Pernambuco
3.2.3 Dimension III – Identification and significance of exporting difficulties
This dimension identifies and classifies the main difficulties faced by the companies to export the products to European Union. A Likert Scale model was utilized to quantify the significance of difficulties, giving values to the categories from 0 to 4.
The median significance of every variable – difficulty faced to export to European Union – shows clearly that the products adequation to the technical standards is the most impacting difficulty, reaching the range “very significant”. In a second range were the difficulties considered “significant”: the product risk analysis; the shipped software validation; the distribution, marketing and technical assistance; and the product price (Figure 1).
Figure 1
Difficulties faced in exportation to European Union
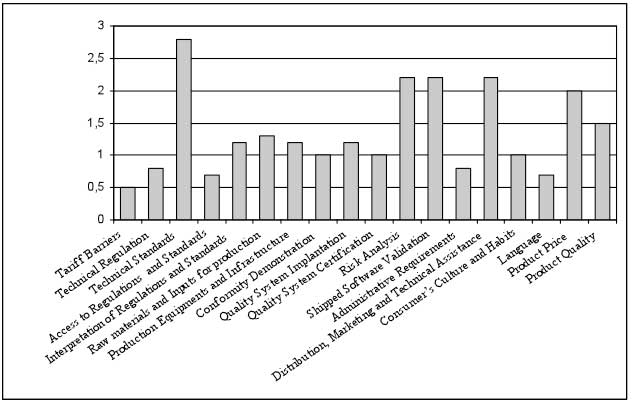
Source: Prepared by the authors
3.2.4 Dimension IV – Investment in research, development and innovation
The survey results demonstrate that the producers have difficulties to identify, to segregate and to account the (financial) resources applied in research, development and innovation. Meanwhile, the companies informed the investments in 2006, in research, development and innovation: from 2% to 20% of annual incomes, and, to the most of the producers, these percentages have been growing in the last five years.
The number of employees working in technical areas of the companies, according to their higher academic grade, is shown on Table 5. The knowledge areas mentioned, according to the grade, are:
Table 5
Number and academic grade of employees working in technical areas of the companies
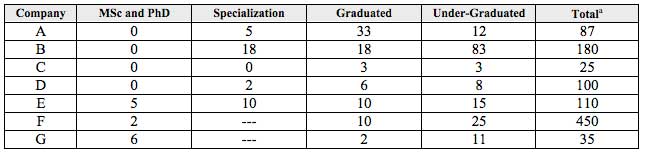
Source: Prepared by the authors
a This total includes the employees which work on the commercial, administrative and operational areas.
The external technical support used by the companies to develop the research, development and innovation activities, basically comes from universities, research centers, technical schools, and certification bodies. To the employees’ formation & training were mentioned: EPUSP, SENAI - National Service for Industrial Learning, and some certification bodies (FCAV – Carlos Alberto Vanzolini Foundation, DNV - Det Norske Veritas, UL - Underwriters Laboratories). To the product and process development, the external technical support is given by IPT, INPE, LABELO, IPEN and SENAI. To the tests and analysis were mentioned: IPT, INPE, NMI, IPEN, HCRP - Clinics Hospital of Ribeirao Preto of the University of São Paulo and UFPE. Most of the companies surveyed received amounts of public founds for research, development and innovation of products, from 2002 to 2006, through government agencies. The programs mentioned were: PROGEX - Program of Technological Support to Export, sponsored by FINEP - Financing Agency for Research and Projects and SEBRAE - Brazilian Micro and Small Business Support Service; PDTI - Industrial Technology Development Program and PAPPE - Support for Company Research Program sponsored by FINEP and PIPE - Small Business Innovation Research Program sponsored by FAPESP- The State of São Paulo Research Foundation.
3.2.5 Dimension V –Factors of Success
The last question of the survey was: which are the most significant factors to the success of the company in overcoming the difficulties faced in exportation process to European Union?
The factors mentioned were: